- Bhagyanath SURESH
Cytomorpholab IPGG
Sharp Transition in MTOC Positioning Mediated by Molecular Motors – In-Vitro
- Baptiste Grimaud, Maxence Frétaud, Feriel Terras, Antoine Bénassy, Karine Duroure, Valérie Bercier, Gaëlle Trippé-Allard, Rabei Mohammedi, Thierry Gacoin, Filippo Del Bene, François Marquier (1), Christelle Langevin, and François Treussart
1 - LUMIN, ENS Paris-Saclay
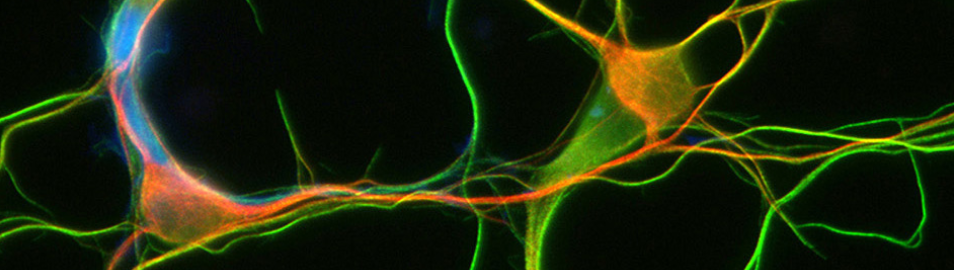
In-vivo fast non-linear microscopy reveals intraneuronal transport impairment induced by slight molecular motor imbalances in the brain of zebrafish larvae
- Maufrant J.(1), Di Cicco A.(1), Battistella A. (1), Manzi J. (1), Levy D. (1), Janke C. (2), Dezi Manuela (1)
1 - Institut Curie, Sorbonne Univ. / 2 - Institut Curie, Univ. Paris-Saclay
In vitro characterization of VAPB-Microtubules interaction by cryo electron microscopy
Abstract: VAPB and its homolog VAPA, are transmembrane proteins members of a conserved family, broadly expressed at the ER. They share the same structure and they interact with hundreds proteins playing a key role in many cellular processes. The identification of a single missense mutation in VAPB gene in a familial ALS8 has triggered an increased interest in understanding the mechanism underlying VAPB function in motor neurons [1]. Moreover, VAP was shown to be responsible of synaptic buttons growing in Drosophila, synaptic vesicles cycling and microtubule (MTs) organization. Our goal is to provide insight into the mechanisms by which VAPB mutation or depletion, can result in specific degeneration of neurons focusing on the interaction with MTs.
Here we describe the VAPB-MTs interaction using un in vitro approach coupled to cryo –Electron Microscopy based on our expertise [2]. We designed an in vitro system using ER-mimicking proteoliposomes and polymerised MTs. Full length VAPB was expressed and purified from E coli and MTs were polymerised in presence of GMPCPP starting from tubulin purified from mouse brain. We first characterised VAPB reconstituted proteoliposomes and verified the specificity of the interaction of polymerised MTs with purified VAPB, using pellet assays. Next, we optimised samples for cryo-Electron Tomography and collected high resolution tomograms. First, images of reconstituted liposomes shown a membrane remodelling effect indicating a membrane curvature preference for VAPB. Second, the high resolution tomograms and the biochemical assay revealed without ambiguity, a specific VAPB dependent interaction. Third, we proved that this interaction exists without any
additional partner protein. In summary this results suggests a that VAPB might contribute in stabilising MT along the neuron axon, helping ER to maintain its structure.
[1] Teuling et al. (2007). Motor Neuron Disease-Associated Mutant Vesicle-Associated Membrane Protein-Associated Protein (VAP) B Recruits Wild-Type VAPs into Endoplasmic Reticulum-Derived Tubular Aggregates J. Neuroscience. 36:9801-9815
[2] De La Mora et al. (2021). Nanoscale architecture of a VAP-A-OSBP tethering complex at membrane contact sites. Nat. Comm. 1:3459
- Marie-Charlotte Emperauger, Florian Semmer, François Treussart, Karen Perronet and François Marquier
LUMIN, ENS Paris-Saclay
Holographic two-photon microscope for real-time 3D single-particle tracking
- Sarah Klein (1,2), Max Ebbinghaus (1,2), Cécile Appert-Rolland (1), Ludger Santen (2)
1 - IJCLab (former LPT), Univ. Paris-Saclay / 2 - Dpt of Physics, Univ. Saarland
Intracellular transport of cargos: tug-of-war, anomalous diffusion, and lattice dynamics